1.0 PURPOSE:
To provide a procedure for stability studies of finished products which includes Receipt of samples, Incubation of sample and withdrawal of sample.
2.0 SCOPE:
This procedure for stability studies of finished products is applicable for all stability samples handled by Quality Control Department which are from exhibit bathes, site transfer batches, contract stability batches and commercial batches.
3.0 DEFINITIONS:
4.0 RESPONSIBILITY:
4.1 Quality assurance personnel to collect the sample from production department and label the stability samples as per the procedure.
4.2 Quality control personnel are responsible for receipt, incubation withdrawal and analysis of stability samples.
5.0 PROCEDURE:
5.1 Receipt of stability samples:
5.1.1 Quality control personnel shall receive stability samples along with approved Stability protocol from QA/Contract provider.
5.1.2 Stability calendar (monthly schedule) shall be prepared as per the approved protocol for the products.
5.1.3 Calculate the pull out dates based on number of days as per the respective stability protocol of individual product.
5.1.4 Calculate the stability sample quantity and pack the samples as per intervals according to approved protocol. Label the outer pack with the label. Print the labels as per below
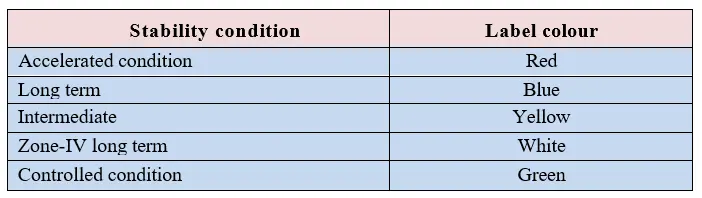
5.1.5 Sample entry details shall be logged into stability sample loading register.
5.2 Incubation of samples:
5.2.1 Ensure samples are stored in respective labeled storage conditions.
5.2.2 Ensure samples are retested for T0 analysis before initiating stability study in case batch is submitted for stability study after 1 month of product release/ Product development.
5.2.3 Charge the samples to stability chambers taking care to place the samples at appropriate orientation mentioned in the respective stability study protocol.
5.2.4 Document the details like receipt/incubation date, product name, quantity received, date of initiation, location of the sample in the stability chamber in the respective issuance receipt register.
5.3 Withdrawal of samples:
5.3.1 Prepare the stability schedule for the next month on the Last week of current month.
5.3.2 This schedule shall be finalized at the last day of the month and approved head quality control or his designee.
5.3.3 The number of sample for testing shall be drawn from the chamber as per the protocol and same shall be mentioned in register.
5.3.4 Personnel responsible for stability analysis shall withdraw the samples from the respective stability chamber and bring the samples to quality control laboratory.
5.3.5 Personnel shall make the entries in the register after withdrawal of the samples.
5.3.6 Time frame for withdrawal of samples +5 days from the due date in case of stability samples up to 6 months and +10 days from the due date for stability samples beyond 6 months.
5.3.7 Withdraw stability samples from the stability chambers as per the respective protocol within the time frame mentioned in the above table.
5.3.8 If there is a deviation in the above time frame in sample withdraw from stability chamber initiate deviation.
5.3.9 Document, if any extra sample issued for any reason (e.g. experimental error, Retesting and additional testing, etc.) It shall be issued from contingency samples.
5.3.10 The withdrawn samples shall be stored at respective labeled storage conditions till the analysis of stability samples.
5.4 Photo stability studies:
5.4.1 Conduct stability on at least one batch. Also, in case of major changes in formulation photo stability studies needs to be repeated as applicable.
5.4.2 Photo stability for finished products shall be decided on case by case. Decision shall be made by designees of FDD, QCD and QA departments.
5.4.3 Carry out the photo stability of product in following conditions.
5.4.4 Tests on the exposed finished product outside primary pack (direct exposed sample) for oral solid dosage forms.
5.4.5 Tests on finished product Covered with aluminum foil (as control sample).
5.4.6 Tests on finished product in the marketing pack (secondary Pack).
5.4.7 For liquid dosage forms direct exposed study shall be performed on primary pack.
5.4.8 The study samples are stored at respective labeled storage condition if the Photo stability chamber is fully loaded with other study samples.
5.4.9 Record the details in photo stability samples receipt, sample size, sample loading and issuance register.
6.0 ABBREVIATIONS:
SOP : Standard Operating Procedure
FDD : Formulation Development Department
QA : Quality assurance
7.0 REFERENCES:
8.0 LIST OF ANNEXURES:
Nil.
