1.0 PURPOSE:
2.0 SCOPE:
This procedure for sampling of In-process and finished products is applicable for sampling of In-process blend, intermediates and finished product manufactured at (Company name).
3.0 DEFINITIONS:
4.0 RESPONSIBILITY:
4.1 Production person to give request to QA for sample collection.
4.2 QA personnel to collect the samples.
4.3 Head – QA to ensure compliance.
5.0 PROCEDURE:
5.1 After receiving the “Test request form” from Production, the concerned IPQA personnel shall fill the sample for analysis label and arrange for sampling aids like clean stainless steel scoop, spoon, sampling thief, Hicoflex sampler/ clean Polybag of suitable size, falcons, sterile bottles of suitable size, etc.
5.2 Check that all the product containers to be sampled bear stage labels.
5.3 For each product stage wise the sample quantity shall be predefined.
5.4 Collection of In-process sample for Oral Solid Dosages:
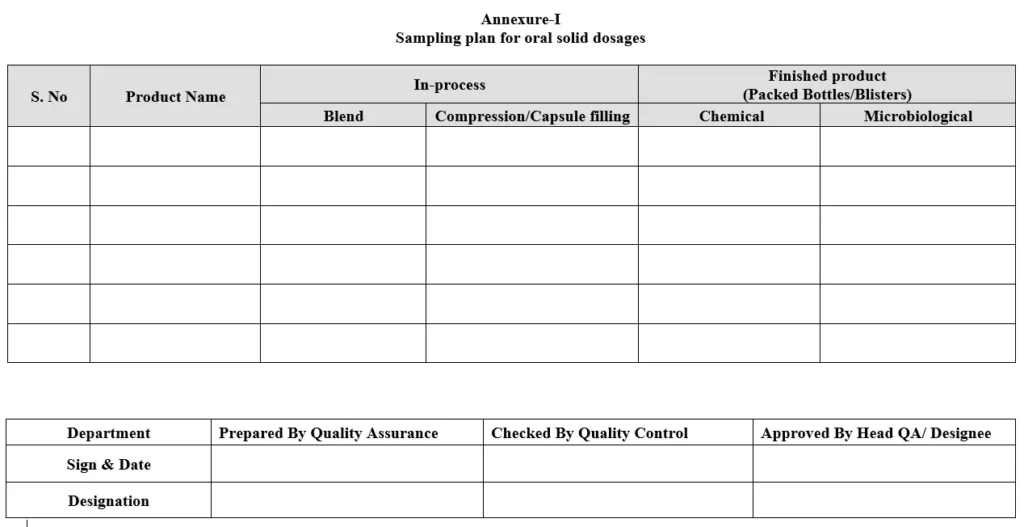
5.4.1 Collect the blend sample with the help of Hicoflex sampler / sampling thief / scoop in desired quantity as per sampling plan for Oral Solid Dosages (Refer Annexure-I). Enter the of sample details in TRF.
5.4.2 Collect the blend uniformity sample prior to composite sample, if any.
5.4.3 Blend uniformity sample shall be collected from different stages like dry mixing/ blending/ lubrication by using unit dose sampler as per respective sampling plan. Composite sample shall be collected from the top, middle, bottom locations of the blend.
Note: If containers/Bins are more than five then samples shall be collected from two locations of the each container/Bin at top and bottom.
5.4.4 Compressed tablets where coating is the final stage then, in process sample of compressed tablets shall be collected as per the sampling plan for Oral Solid Dosages (Refer Annexure-I) or as per the protocol whichever is applicable.
5.5 Collection of Finished product samples:
5.5.1 For uncoated tablets / coated tablets / filled capsules; required sample shall be collected as per quantity mentioned in sampling plan for Oral Solid Dosages (Refer Annexure-I) or as per the protocol whichever is applicable.
5.5.2 The finished product sample shall be collected from all the containers equally and make a composite sample.
5.5.3 Note: If containers are more than five then samples shall be collected from two locations of the each container/Bin at top and bottom.
5.6 Samples for Microbiological analysis for Tablet and Capsules:
5.6.1 Samples for microbiological analysis shall be collected after packing as per specification if required. The sample shall be collected as per the quantity mentioned in the sampling plan for Oral Solid Dosages (Refer Annexure-I).
5.6.2 If sampling performed by breaking the containment then, it shall be collected by wearing pressure suite.
5.6.3 Samples shall be collected as per the protocol or sampling plan for Oral Solid Dosages (Refer Annexure-I) whichever is applicable.
5.7 Sampling details for Injectables:
5.7.1 Sampling from compounding stage:
5.7.1.1 After collection of WFI into the compounding vessel, collect 10 ml WFI sample for BET in a depyrogenated sampling container and 100ml WFI sample shall be collected in a cleaned container for chemical analysis from the bottom valve of compounding vessel. Label the container properly and send the sample to QC with TRF for analysis.
5.7.1.2 After completion of compounding process collect 50 ml unfiltered bulk sample in a cleaned sampling container from the bottom valve of manufacturing tank for chemical analysis and collect100 mL of unfiltered bulk sample, in a pre-sterilized sampling container for bio burden analysis. Label the container with sample for analysis and send the sample to QC with TRF.
5.7.2 In case of process validation batch collect unfiltered bulk sample from top of manufacturing tank with liquid sampler under continuous stirring as per sampling plan. Collect bottom sample through bottom valve of manufacturing tank.
5.7.3 In case of hold time study collect the unfiltered and filtered bulk sample as per sampling plan. Collect sample through bottom valve of manufacturing and filtration tank.
5.7.4 For filtered bulk collect in process sample as per sampling plan.
5.7.5 After sample collection wrap the container with aluminium foil and paste the sample for testing label.
5.8 Sampling procedure for filled & sealed vials:
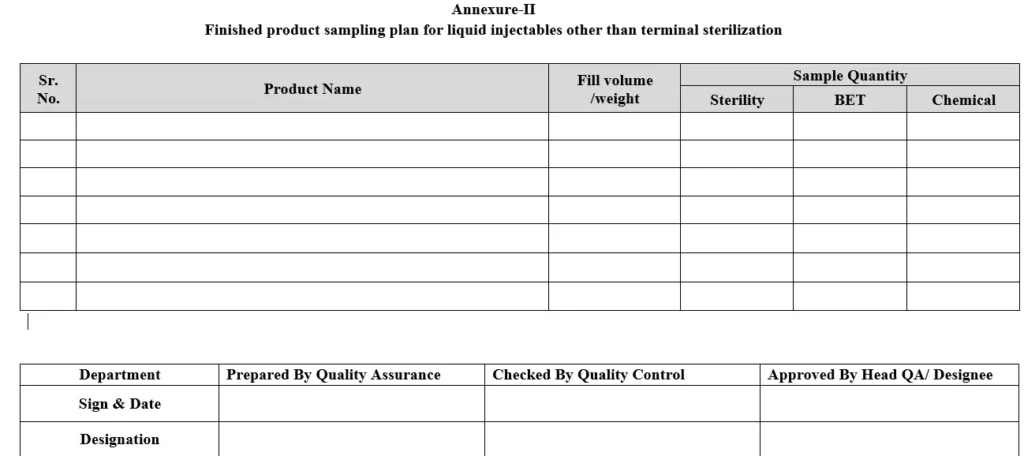
5.8.1 Composite sample of filled & sealed vials shall be collected after external vial washing & batch coding at regular intervals (initial, middle & end of the batch coding). Equivalent quantity as per sampling plan shall be collected from each interval. Refer sample quantity as per Annexure-II (Finished product sampling plan for Liquid Injectables other than terminal sterilization).
5.9 Sampling procedure for Lyophilized filled & sealed vials other than terminal sterilization:
5.9.1 Composite sample of filled & sealed vials shall be collected after external vial washing & batch coding at regular intervals (initial, middle & end of the batch coding). Equivalent quantity as per sampling plan shall be collected from each interval. Refer sample quantity as per Annexure-III for “Finished product sampling plan for Lyophilized products”.
5.10 Sampling procedure terminally sterilized filled & sealed vials:
5.10.1 After completion of terminal sterilization cycle for sterility, samples shall be collected from each terminal sterilization load. Refer sample quantity as mentioned in Annexure-IV for “Finished product sampling plan for terminally sterilized products”. Sterility sample shall be collected in the separate poly bag and label the polybag as per each terminal sterilization load.
5.10.2 For chemical, composite sample shall be collected from each load of terminal sterilization. Equivalent quantity of sample shall be collected from the each load, pull the sample and label properly. Refer sample quantity as mentioned in Annexure-IV for “Finished product sampling plan for terminally sterilized products”.
5.11 Note: for exhibit and validation batches or protocol based batches sampling plan shall be prepared and sample quantity shall mentioned in protocol.
5.12 QA personnel shall separately identify the samples for chemical & microbiological testing in the samples for testing label.
5.13 QA personnel shall seal the samples and match the details of the request with respective BMR and enter the sample quantity, sign and date in the requisition form and BMR.
5.14 Sampling plan for solid dosages and injection shall be prepared and updated whenever new product introduced to facility and as when required.
6.0 ABBREVIATION:
6.1 QA : Quality Assurance.
6.2 QC : Quality Control
6.3 BET : Bacterial Endotoxin Test
6.4 TRF : Test request form
7.0 REFERENCES:
8.0 ENCLOSURES:
Annexure-I: Sampling Plan for Oral Solid Dosages
Annexure-II: Finished product sampling plan for Liquid Injectables other than terminal sterilization.
Annexure-III: Finished product sampling plan for Lyophilized products.
Annexure-IV: Finished product sampling plan for terminally sterilized products.