1.0 PURPOSE:
2.0 SCOPE:
The SOP for preparation of protocol and report is applicable to all protocols & reports used at (Company Name).
3.0 DEFINITION:
Qualification or validation protocol: It is an authorized written document specifying the procedure for carrying the qualification and validations.
Qualification or validation report: It is an authorized written document specifying the procedure which was performed during the qualification or validation activity which includes the data generated during activity.
4.0 RESPONSIBILITY
Execution: Officer QA or Related Department; Checking: Officer /Executive QA; Accountability: Head QA.
5.0 PROCEDURE:
5.1 Numbering system of protocols and reports:
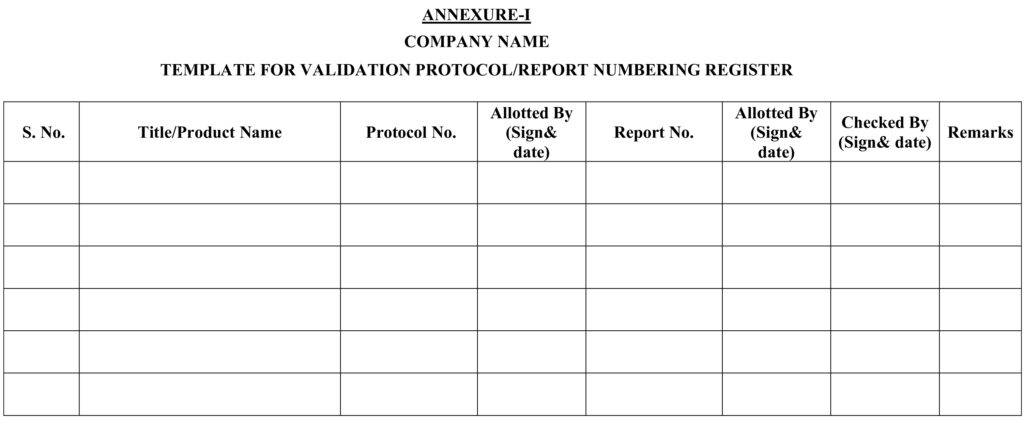
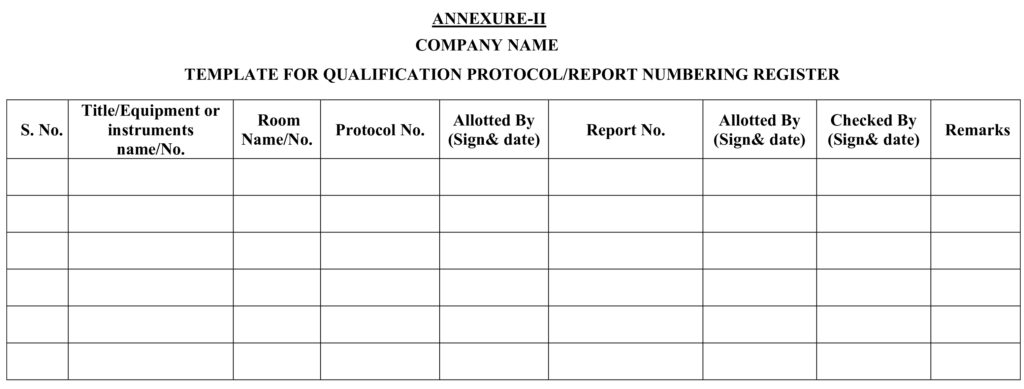
5.1.1 QA shall assign all protocols/reports number to all concerned departments and record in same as per Annexure-I for validation activity and Annexure-II for qualification activity.
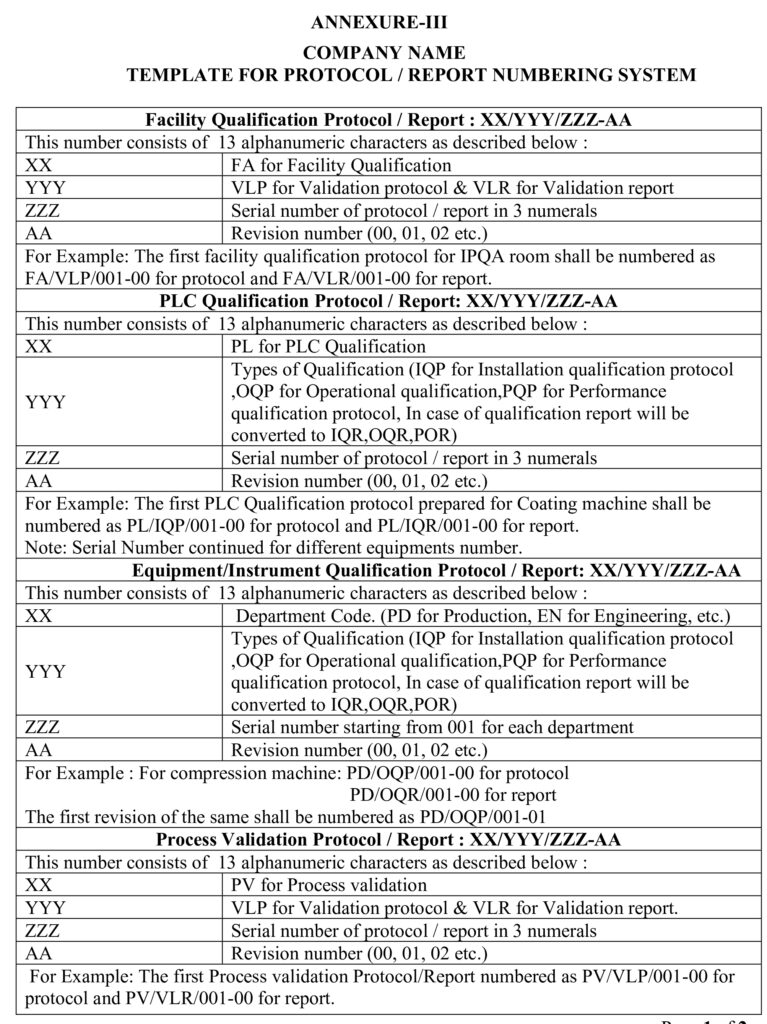
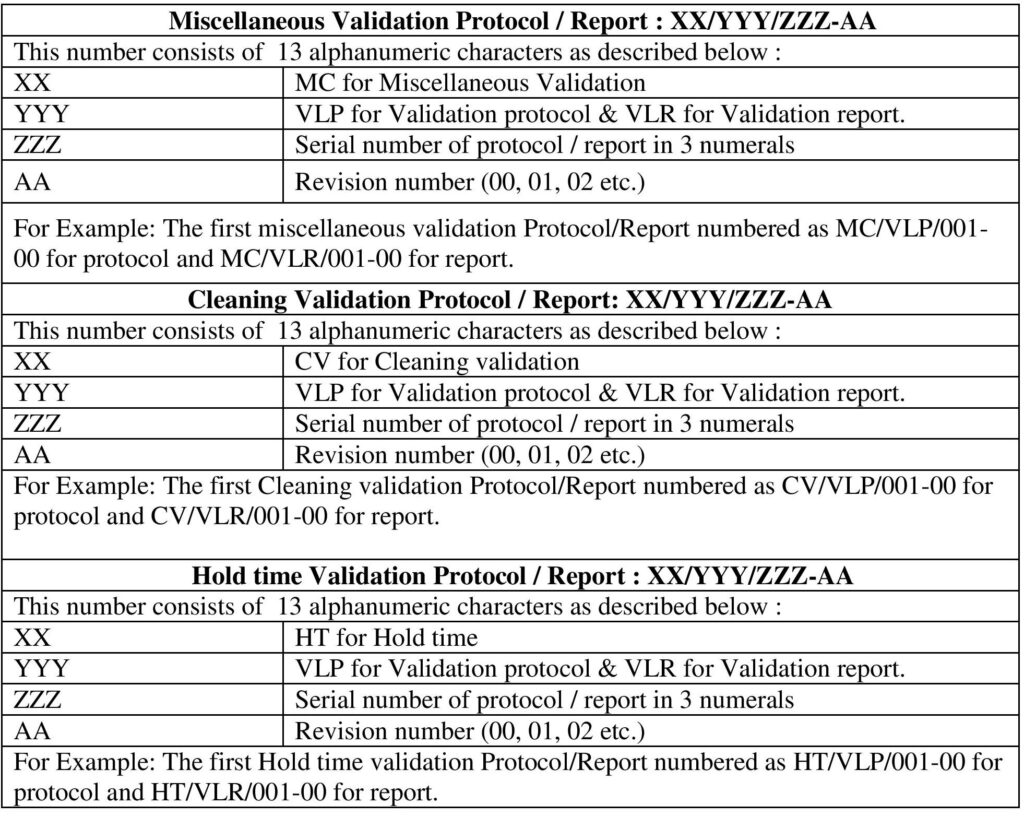
5.1.2 Protocol number shall be given as per the Annexure-III.
5.2 Preparation and approval of protocol:
5.2.1 Head of the user department shall inform to the concern chemist / officer / executive for the preparation of protocol.
5.2.2 The responsible person shall prepare the protocol using the below mentioned specifications and guidance provided by the department Head and Head-QA.
5.2.3 The specifications for all protocols shall be preferably as mentioned in the protocol. Minor alterations in specifications specified in protocol may be accepted in compulsory situations with prior acceptance by Head-QA.
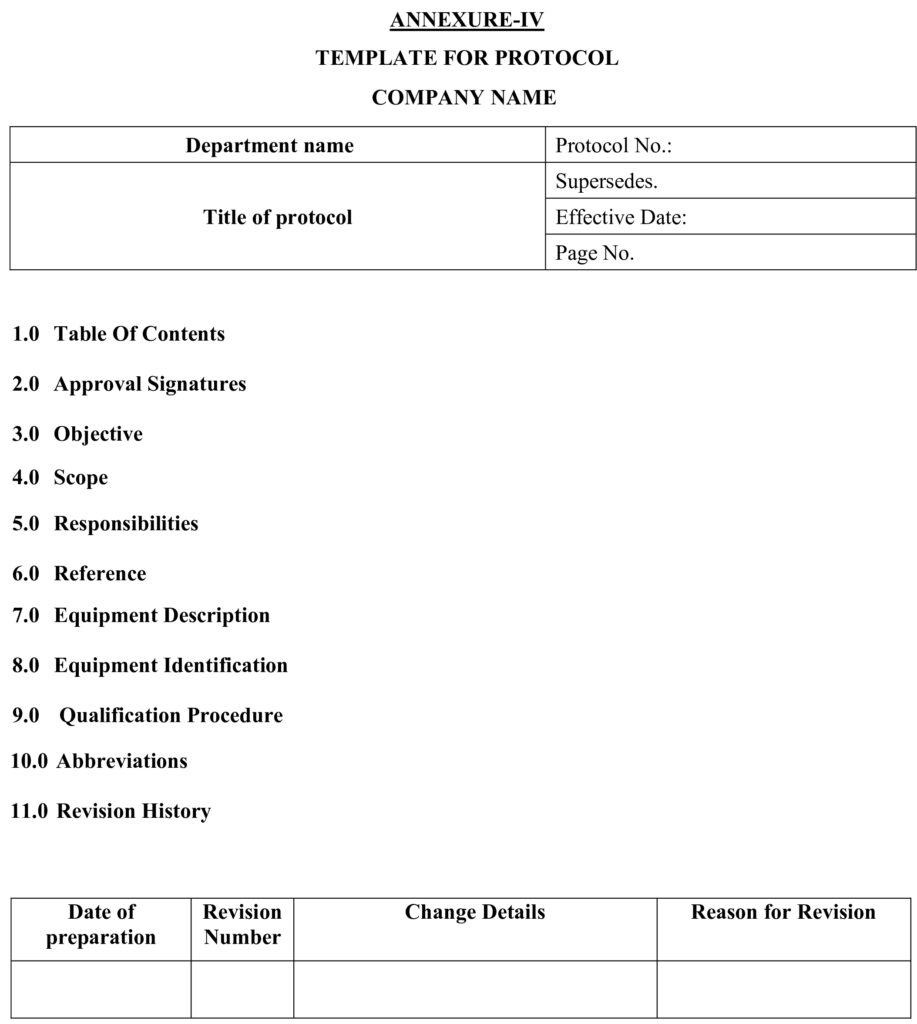
5.2.4 Prepare the protocol as per the template given as Annexure-IV.
5.2.5 Protocols shall be prepared using the following as references as applicable:
5.2.5.1 Manuals, technical documents provided by vendors, design specifications and qualifications, GMP requirements / regulations, GMP guidelines, compendia requirements, process requirements etc.
5.2.6 If the protocol initially prepared and same protocol shall be revised (if applicable), then number the protocol as per Annexure-III and initial protocol shall be supersedes.
5.2.7 Any changes shall be handled through a “change control”, and updated in the change history sheet.
5.2.8 When requalification/revalidation shall be performed in this case protocol shall be revised (if required).
5.2.9 After preparation, initial review shall be done by QA followed by other department heads based on the type of protocol.
5.2.10 Finally Head-QA shall review for its completeness, correctness and compliance with the GMP requirements and regulations and approve the protocol as appropriate. Each page of the approved protocol shall be stamped MASTER COPY.
5.3 Issue of protocol and execution:
5.3.1 After approval the controlled copy of protocol shall be issued to the concern department for execution by photocopying the master protocol.
5.3.2 Concern department shall execute the protocol for qualification or validation in co-ordination with other members of execution team as specified in the qualification or validation protocol.
5.3.3 As a member of execution team one of the members from QA shall monitor all the qualification or validation activity.
5.4 Preparation and approval of report:
5.4.1 After completion of the qualification or validation activity chemist / officer / executive of the concern department shall prepare the qualification or validation report as per Annexure-V. Report number shall be given as per the Annexure-III.
5.4.2 He shall compile all the data related to the qualification or validation and attach to the report and submit to QA.
5.4.3 After preparation of report, initial review shall be done by QA followed by other department heads based on the type of qualification or validation.
5.4.4 Finally Head-QA shall review for its completeness, correctness and compliance with the GMP requirements and regulations and approve the report as appropriate.
5.5 Master copy of protocol and report shall be retained by QA.
6.0 ABBREVIATIOS:
SOP: Standard operating procedure
QA : Quality Assurance
GMP: Good Manufacturing Practices
No. : Number
S. No.: Serial Number
7.0 REFERENCE:
8.0 ENCLOSURES:
Annexure-I : Template Validation protocol/report numbering register
Annexure-II : Template Qualification protocol/report numbering register
Annexure-III: Template Protocol / Report numbering System
Annexure-IV: Template for protocol
Annexure-V : Template for report


4 thoughts on “SOP for preparation of protocol and report”
Pingback: Preparation, review and approval of facility qualification -
Pingback: SOP for process validation of pharmaceutical products -
Pingback: SOP for reworking or reprocessing of pharmaceutical product -
Pingback: Cleaning Validation protocol -