1.0 PURPOSE:
To lay down a procedure for Operation and cleaning of Tablet Hardness Tester
2.0 SCOPE:
This procedure covers the operation and cleaning of Tablet Hardness Tester, which is used in Quality Assurance department of (Company name).
3.0 DEFINITIONS:
Nil.
4.0 RESPONSIBILITY:
4.1 QA personal is responsible for operation of Tablet Hardness Tester as per the SOP of the instrument.
4.2 Head-QA or designee is responsible for ensuring the compliance of the procedure.
5.0 PROCEDURE:
5.1 Check and ensure that the instrument is clean if not clean, clean it with the soft brush and lint free cloth.
5.2 Verify the calibration status of the instrument for its validity.
5.3 Bring the jaw to the zero position and switch on the instrument by pressing ON/ZERO key wait till initialization completes.
5.4 Select the required unit as per requirement by pressing UNITS key.
5.5 Bring the jaws apart by moving the knob clockwise direction.
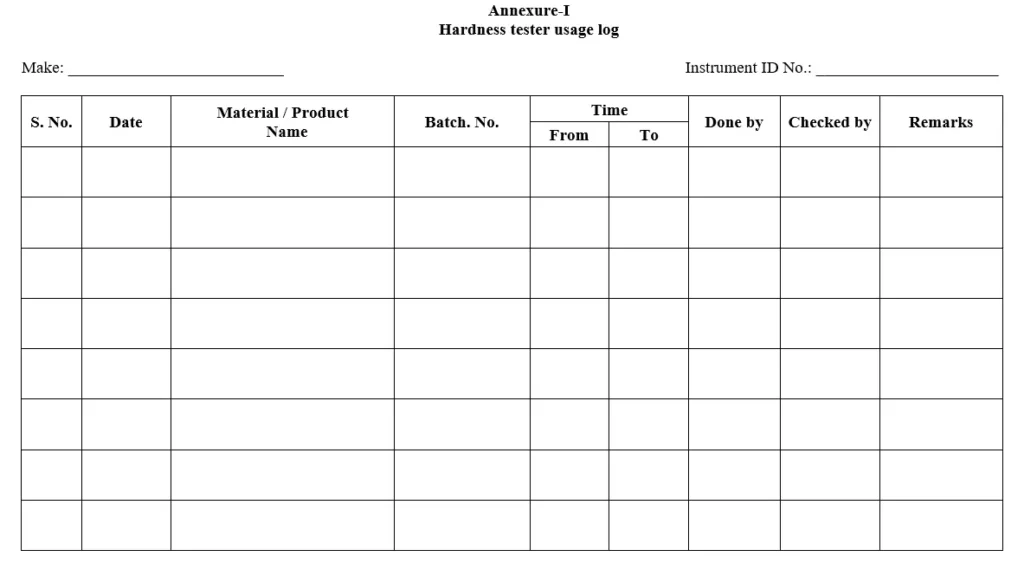
5.6 Place the tablet between the jaws of the hardness tester and turn the knob slowly in anticlockwise direction until the tablet breaks note down the reading as per Annexure-I and clean the instrument with the soft brush.
5.7 Repeat the operation 5.3 to 5.6 for multiple determinations.
5.8 Cleaning:
5.8.1 Clean the Hardness tester with dry lint free cloth.
5.8.2 The Hardness tester shall be cleaned before and after use using dry Lint free cloth.
5.8.3 Do not use any solvent for the cleaning.
5.8.4 Do not use compressed air for cleaning.
6.0 ABBREVIATIONS:
SOP : Standard Operating Procedure.
QA : Quality Assurance
7.0 REFERENCES:
Nil.
8.0 LIST OF ANNEXURS:
Annexure-I: Hardness tester Usage log book.