1.0 PURPOSE:
2.0 SCOPE:
3.0 DEFINITIONS:
3.1 Retest: Examine the material for its physical and / or chemical properties after definite time interval for its suitability for usage, which is stored under specified storage conditions.
3.2 Expiry: It is a date or period, after that material is not suitable for use.
4.0 RESPONSIBILITY:
4.1 Warehouse personnel are responsible for identification and control of materials due for retest and expiry and providing intimation of the same to QC, QA, Production and Purchase department.
4.2 QC personnel are responsible for re-sampling and subsequent analysis of materials due for retesting.
4.3 Head-Warehouse / Designee are responsible for compliance of this SOP.
5.0 PROCEDURE:
5.1 Identification and Control of Materials due for Retest:
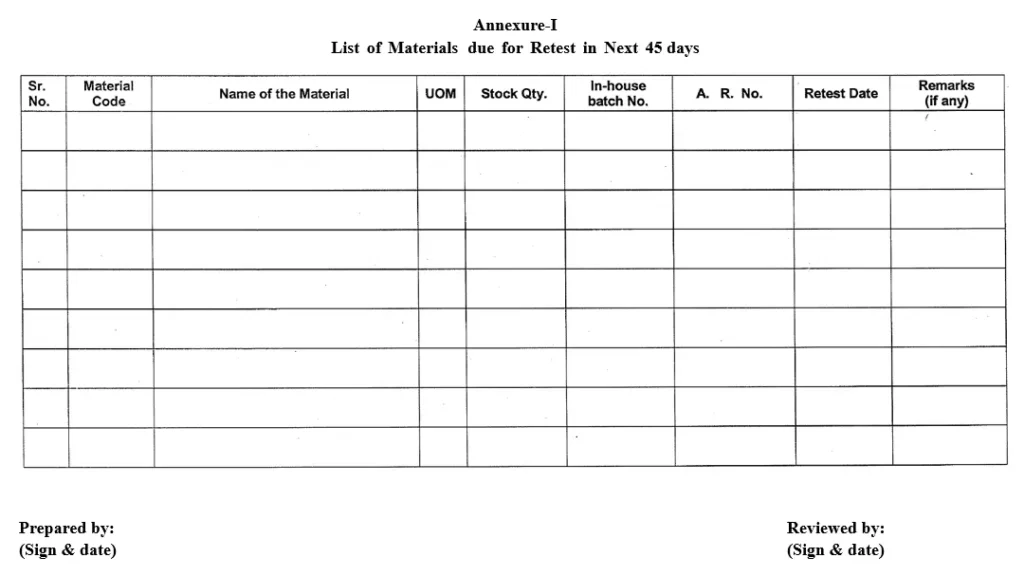
5.1.1 First week of every month, warehouse personnel shall prepare a list as “List of Materials due for Retest in Next 45 days” as per Annexure-I by referring SAP and display it.
5.1.2 Warehouse person shall inform about the “List of Materials due for Retest in Next 45 days” to Production, QC, QA and Purchase department for necessary action.
5.1.3 File the previous months “List of Materials due for Retest in Next 45 days” in office-file.
5.1.4 Daily warehouse personnel shall refer the “List of Materials due for Retest in Next 45 days” as per Annexure-I.
5.1.5 The material can be used / consumed or sampled in advance before the retest due date. The material should not be used / consumed on or after retest date unless analyze as per specifications.
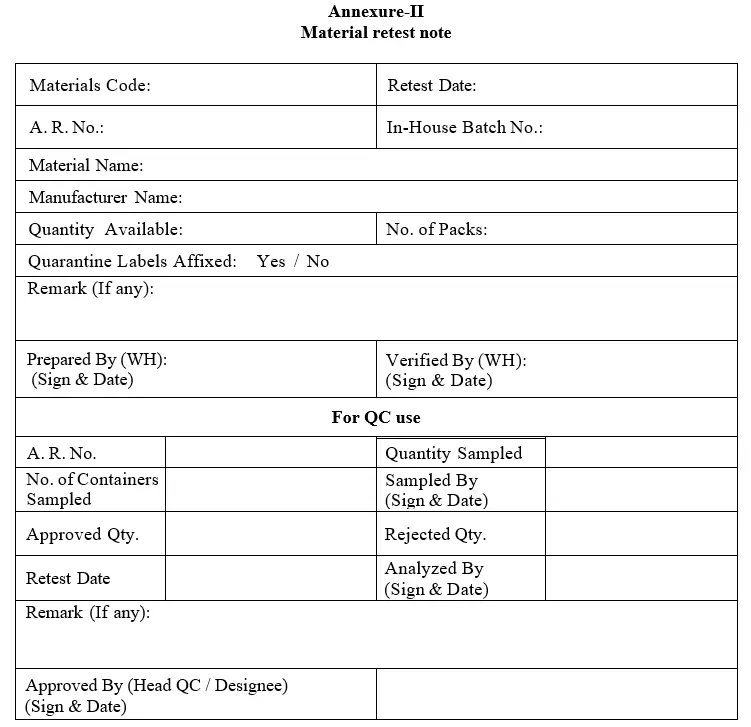
5.1.6 On the day of retest date, warehouse person shall raise “Material Retest Note” as per Annexure-II for identified materials and paste “Quarantine (Under Retesting)” labels on each container / pack by defacing the previously affixed approved label and the material shall be physically transferred to quarantine area and should not be used until retesting. Such materials shall be sampled and tested based on production priority.
5.1.7 As per production planning, production person should intimate to Warehouse and QC in advance for performing retesting of materials due for retest based on priority. In such case Warehouse person shall generate “Material Retest Note” and handover it to QC for performing sampling. QC shall sample the material from Approved stock if the material is still in validity for retesting or from Quarantine if the material is already due for retest. QC shall affix sampled label by defacing previous sampled label.
5.1.8 After sampling of the material for retest, sample quantity shall be updated in SAP.
5.1.9 After sampling, approved material shall be stored back in Approved material storage rack on green pallet and can be used as per FEFO / FIFO before retest due date.
5.1.10 After completion of QC analysis, QC will intimate to Warehouse for generation of “Quarantine (Under Retesting)” labels for the materials available in Approved stock.
5.1.11 Warehouse person shall remove material pallet from Approved storage racks, affix Quarantine (Under Retesting) label by defacing previous Approved status label, simultaneously QC will affix the Approved label on Quarantine (Under Retesting) label and handover the original copy of “Material Retest Note” i. e. white copy to Warehouse.
5.1.12 Warehouse person shall again shift the material in Approved Storage racks as per storage condition.
5.1.13 If material gets rejected after analysis, QC should affix Rejected labels to each container / pack over the Quarantine (Under Retesting) label. Warehouse person shall immediately transfer the material containers from approved storage racks to rejected area of respective storage condition.
5.1.14 If QC unable to provide the results till actual retest date of material, then on the day of actual retest date warehouse person shall transfer the material containers from Approved Area to Quarantine Area by affixing “Quarantine (Under Retesting)” label.
5.1.15 Warehouse person shall track the materials retest notes submitted to QC, by putting a remark in “List of Materials due for Retest in Next 45 days”.
5.1.16 No material shall be retested after the shelf-life / expiry date of the material.
5.2 Identification and Control of Materials Due for Expiry:
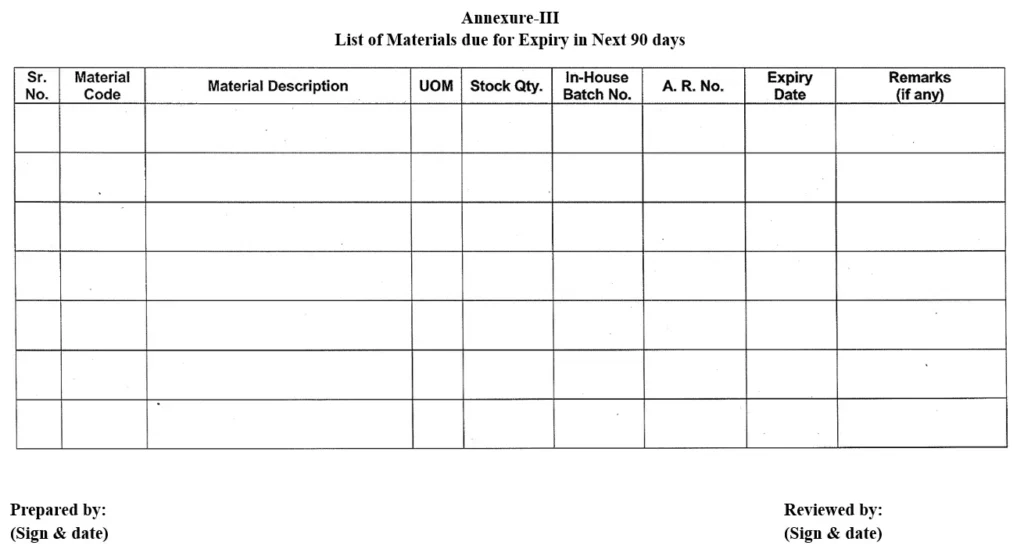
5.2.1 First week of every month, Warehouse personnel shall prepare a list as “List of Materials Due for Expire in Next 90 days” as per Annexure-III referring material SAP and display it.
5.2.2 Warehouse person shall inform about the “List of Materials due for Expiry in Next 90 days” to Production, QC, QA and Purchase department for necessary action.
5.2.3 File the previous months “List of Materials Due for Expiry in Next 90 days” in office-file.
5.2.4 Daily warehouse personnel shall refer the “List of Materials Due for Expiry in Next 90 days” as per Annexure-III.
5.2.5 Production department shall evaluate the usage of material before expiration.
5.2.6 If any material is found to be due for expiry, on the day of expiry warehouse person shall affix the “Expired Material” label and shift the material containers to respective storage condition rejection area.
6.0 ABBREVIATIONS:
SOP – Standard operating procedure.
QA – Quality assurance
QC – Quality control
SAP – Systems Applications and Products in Data Processing
FIFO – First in first out
FEFO – First expiry first out
7.0 REFERENCES:
8.0 LIST OF ANNEXURES:
Annexure-I: List of Materials due for Retest in Next 45 days.
Annexure-II: Material Retest Note.
Annexure-III: List of Materials due for Expiry in Next 90 days.