1.0 PURPOSE:
2.0 SCOPE:
This procedure for handling of temporary change control is applicable to all departments in (Company name). The scope is extended to any temporary change planned in the following aspects (but not limited to):
2.1 Quality Policy and Management System
2.2 Facilities, construction and HVAC
2.3 Machinery and equipment
2.4 Materials usage
2.5 Process
2.6 Approved SOP’s, specifications and any approved documents.
2.7 Any other temporary change which may affect product quality.
3.0 REFERENCE:
4.0 DEFINITION:
Temporary change: Deviations in the established systems, standardized procedures and processes, deliberately made and planned in advance, in order to improve upon product quality, ease of operation, cost effectiveness, time and manpower saving etc. are temporary change.
5.0 RESPONSIBILITY:
5.1 Initiator: Personnel of concerned department is responsible for initiation of temporary control.
5.2 Department Head/designee: To ensure the implementation and closing of temporary change control.
5.3 Head-QA: Check the adequacy of the justification provided, Review the impact of the changes and approval / rejection of change proposed.
6.0 PROCEDURE:
6.1 Whenever a temporary change is planned / required, respective department shall request for “Temporary change form”.
6.2 Temporary change form is applicable for a specific number of batches / specific period.
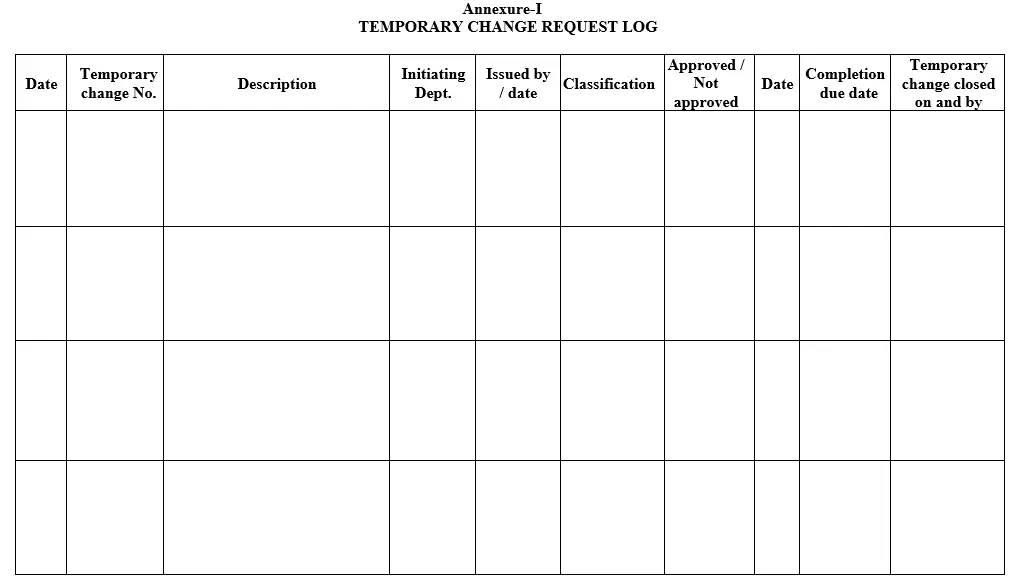
6.3 On the receipt of the temporary change request form concerned department, QA shall register the temporary change after entering the details as specified in temporary change request log as per Annexure-I and allocate the number as detail below.
6.4 Temporary change request number shall be allotted as TC/XXX/YY/ZZZ, in which,
TC – Indicates Temporary Change
XXX – Indicates first three letter of company name.
YY – Indicates the last two numerical of the current year
ZZZ – Indicates the serial number of Temporary change (TC) allotted in the
Current year (starting from 001, 002 and so on).
6.5 QA personnel shall issue the temporary change request form as per Annexure-II by filling relevant information as per received requisition.
6.6 Initiator shall write the description of the existing procedure and propose temporary change.
6.7 Head/Designee of initiating department shall write the justification of the Temporary Change.
6.8 Initiating department has to perform the impact analysis of proposed Temporary change in coordination with applicable departments along with QA.
6.9 After completion of impact analysis, initiating department Head/Designee shall propose the corrective actions and preventive actions in consent with applicable departments and QA.
6.10 QA shall review and classify the Temporary change as Minor and Major based on the nature.
Minor Temporary change: Temporary change, unlikely to have impact on quality of the product, it shall be addressed as per cGMP practices
Examples (But not limited):
Alternate qualified instruments to be used.
Raw materials to be released with partial analysis.
Other alternate approved vendor for secondary and tertiary packaging materials.
Calibrations / Re-Qualifications not completed within the due date and respective equipment / instrument kept under hold without usage.
Major Temporary change: Temporary change which have a moderate impact on quality of the product, corrective actions are required to eliminate the impact.
Examples (But not limited):
Alternate, like to like equipment usage.
After facility renovation, proceeding for manufacturing / packing, before receipt of the complete results and / or completion of qualification report.
Note:
Classification may vary case to case based on impact of the change.
Temporary changes which have substantial impact on quality of the product, such as critical temporary changes shall be rejected.
6.11 QA shall comment and identify the applicable departments for reviewing the temporary change form. Head/Designee of applicable department shall comment on the temporary change form.
6.12 Temporary change shall be communicated to contract giver if required.
6.13 Head-QA/Designee shall review the temporary change request form and approved / not approved (reject) based on the impact.
6.14 If the temporary change request is not approved (rejected), achieve the temporary change request form with reason for rejection.
6.15 After approval of temporary change request form, implementation shall be done by respective departments.
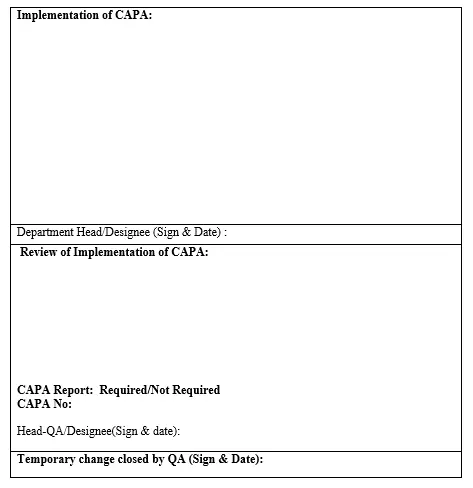
6.16 Implementation details shall be filled in the Temporary change request form by initiating department Head/Designee along with supporting documents.
6.17 Head-QA/Designee shall review the implementation details of temporary change and comment for closure.
6.18 After review comment by Head-QA/Designee, Temporary change request form shall be closed by QA.
6.19 Head–QA/Designee shall suggest the concerned department to initiate the separate CAPA report based on the implementation of CAPA (if applicable).
6.20 If required / suggested by Head-QA/ designee, Temporary change can be converted to permanent change with justification through change control procedure and close the Temporary change.
6.21 Temporary change request form shall be closed within 30 calendar days from date of issuance.
6.22 If the proposed temporary change is not implemented within 30 calendar days, Head/Designee of initiating department shall request for extension of target completion date with justification and shall provide the extended TCD.
6.23 Head-QA/ designee has to approve / reject the extension request by reviewing the reason and impact on ongoing process or product quality.
6.24 Photocopy of Temporary change shall be attached to applicable documents (BPR’s, Protocols, Reports, Record of Results and so on).
6.25 Product quality related Major temporary changes shall be indicated in the Annual product quality review.
7.0 ABBREVIATIONS:
TC : Temporary Change
CAPA : Corrective And Preventive Action
cGMP : Current Good Manufacturing Practice
QA : Quality Assurance
BPR : Batch Processing Record
TCD : Target completion date
8.0 ENCLOSURES:
Annexure-I: Temporary change request log
Annexure-II: Temporary change request form



Also read: SOP for Handling of deviation
Also read: SOP for Change control management
