1.0 PURPOSE:
2.0 SCOPE:
The SOP for handling of returned drug products is applicable to handling of returned drug products (Company name).
3.0 DEFINITION:
Returned Drug Product: A particular batch or product distributed in market for sale and returned to manufacturing location in account of following:
i. Exceeding expiry period.
ii. Damaged goods.
iii. Product not meeting the requirements at the customer end.
4.0 RESPONSIBILITY:
4.1 Officer QA and warehouse is responsible for execution of the procedure.
4.2 Head QA is responsible for overall compliance of the procedure.
5.0 PROCEDURE:
5.1 On authorization from Director-Operations regarding product return, Head-Marketing/Distribution shall organize to transfer the goods to manufacturing location along with a copy of product return authorization.
5.2 Head-Warehouse shall receive the goods and check the delivery Challan and excise invoice details.
5.3 The returned goods shall be stored in specified area de-marked separately with status boards indicating ‘returned goods’.
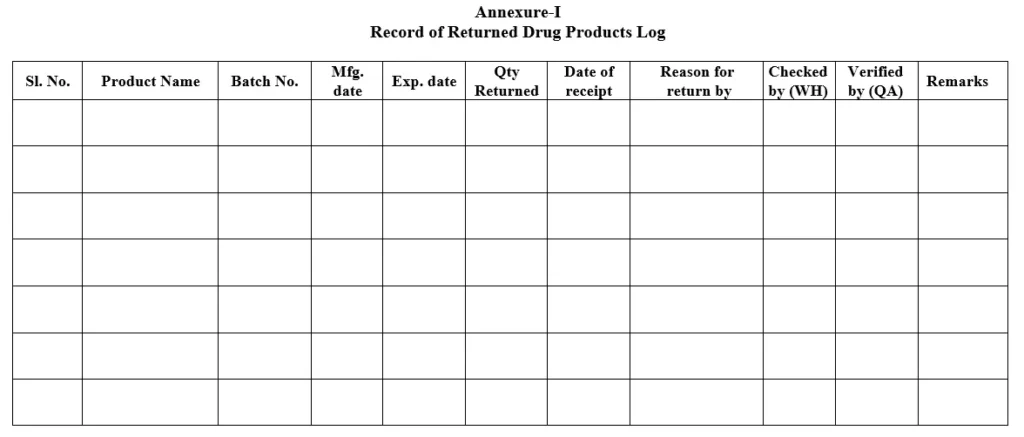
5.4 Record of returned drug product shall be filled as per Annexure-I with the following details:
i. Date of receipt at Warehouse.
ii. The name of the product
iii. Batch number.
iv. Mfg. date
v. Exp. Date
vi. Reason for return.
vii. Quantity returned.
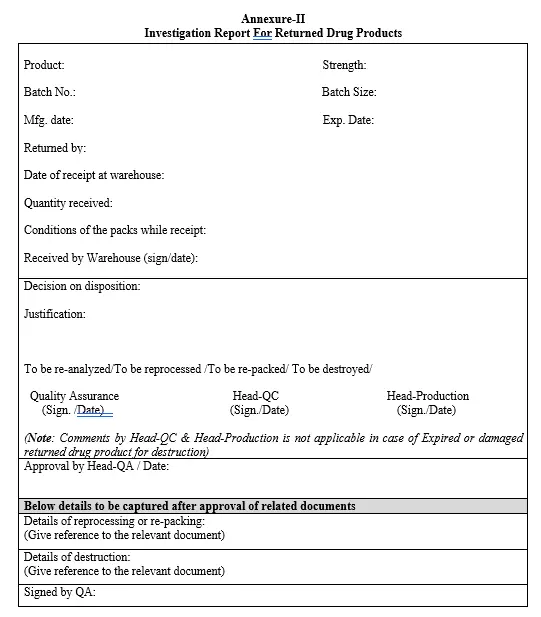
5.5 Investigation report shall be prepared as per Annexure-II by warehouse with required product details. QA shall comment on decision of disposition (to be re-analyzed/repacked/destroyed) with the reason for return. Head-QC and Head-Production shall comments on decision of disposition if applicable.
5.6 Investigation report shall be finally approved by Head-QA.
5.7 Based on the report, disposition of the goods shall be decided whether to repack or salvage or destroy.
5.8 If the condition of drug product, its container, carton or labeling impact the safety, identity, strength, quality or purity of drug product, the returned product shall be destroyed.
5.9 The decision on salvaging or re-packing and return of repacked product into market shall be carried out only, if the drug product meets appropriate standards of safety, identity, strength, quality or purity upon investigation and testing.
5.10 In case of opening primary packaging material (risk of product exposure) testing shall be carried out before repacking or on repacked product as applicable. However release of repacked product into the market shall be approved by Head-QA after completion of complete analysis and compliance as per specification.
5.11 In case of secondary (Non product exposure) if the physical appearance of product/container/blister/vial is in good condition, then the product can be repacked without testing.
5.12 Drug products that have been subjected to improper storage conditions including extremes in temperature, humidity, smoke, fumes, and damage due to natural disastrous, fires, accidents or equipment failures shall not be salvaged and returned to market place.
5.13 Repacking of returned Drug product:
5.13.1 Re-packing shall be carried out after approval of investigation report and related QMS document by Head-QA.
5.13.2 Repacking shall be carried as per approved documents or BPR (as applicable).
5.13.3 Re-packing shall be carried out as per the packing instructions and packing materials for the particular product. All the relevant details of re-packing shall be recorded.
5.13.4 The returned drug products shall be repacked as given below.
5.13.4.1 Production personnel production department shall raise the indent for the required packing material and shall request to QA for issuance of particular BPR or repacking record. QA personnel shall issue the BPR/repacking record.
5.13.4.2 Warehouse personnel shall issue the returned drug products and required packing material to the Production department.
5.13.4.3 Re-packing operation shall be carried out as per the BPR/repacking record.
5.13.4.4 Repacked sampled shall be forwarded to QC for analysis (as applicable).
5.13.4.5 After completion of re-packing, finished product shall be transferred to finished product storage area.
5.14 Release of repacked product shall be done after authorization from Head-QA.
5.15 Head-QA shall inform concerned regulatory authorities through Regulatory Affairs department, in writing, to the pertaining to returned goods as applicable.
5.16 Head-Warehouse shall inform, to Central Excise Department, regarding the details of returned goods as applicable.
5.17 Destruction of rejected returned goods.
5.17.1 Destruction of rejected returned good shall be carried as per SOP of procedure for storage, handling and destruction of rejected / expired materials.
5.17.2 Record the details of destruction as per SOP.
6.0 ABBREVIATIONS:
QA : Quality Assurance
No. : Number
QC: Quality control
7.0 ENCLOSURES:
Annexure-I: Record of Returned Drug Products Log
Annexure-II: Investigation report for returned drug products
8.0 REFERENCES: