Principle:
The limit test for sulphate based on the interaction between soluble sulphate and barium chloride in the presence of dilute hydrochloric acid. This reaction produces barium sulphate, which manifests as solid particles (turbidity) within the solution.

The solution becomes turbid as a result of precipitate formation. The the degree of turbidity dependent on the quantity of sulphate present. If the turbidity produced by the test sample less than of standard solution, it indicates that the sample contains sulfates within the specified limits.
Apparatus required:
1. Nessler cylinder
2. Glass rod
3. Measuring cylinder
4. Pipette
5. Rubber stopper
6. Dropper
Chemicals required:
1. Dilute hydrochloric acid
2. 0.5 M Barium chloride: 122.1 g of BaCl₂.2H₂O dissolved in distilled water.
3. To prepare barium sulphate reagent mix 15 ml of 0.5 M barium chloride, 55 ml of water, and 20 ml of sulphate-free alcohol. Add 5 ml of 0.0181% w/v potassium sulphate to the mixture. Dilute the solution to 1000 ml with water and mix thoroughly.
Procedure for limit test for sulphate:
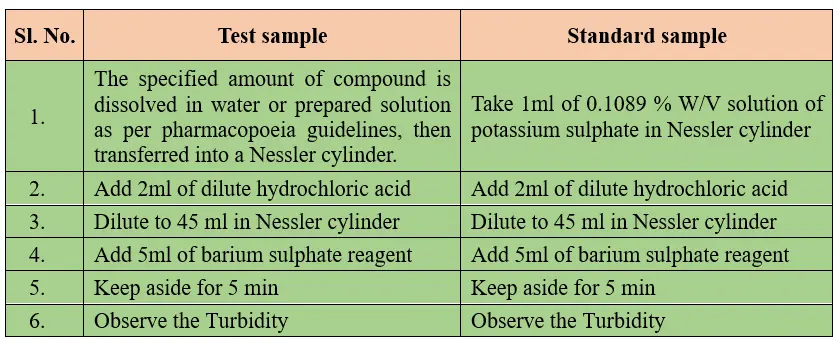
Barium sulphate reagent comprises barium chloride, alcohol free sulphate and small quantity of potassium sulphate.
Observation:
A comparisons study shall be performed visually for both test and standard sample. The turbidity produce in test solution should not exceeds that of standard solution. Turbidity produces in test solution is less than the standard solution indicates pass the limit test for sulphate and turbidity produces in test solution greater than the standard solution indicates fails the limit test.
Reasons:
In limit test for sulphate, Potassium sulphate is used to enhance the sensitivity of the test by providing additional ionic concentration in the reagent, while alcohol aids in preventing supersaturation.
