Principle:
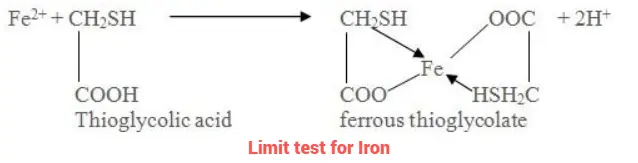
The limit test for iron is based on the formation of the purple color through interaction of iron with thioglycolic acid within a solution buffered with ammonium citrate. This resultant produced is then compared with a reference standard with a predetermined iron content (0.14 mg of Fe).
Apparatus Required in the limit for iron:
1. Nessler cylinders
2. Glass rod
3. Pipettes
4. Stand
Chemicals Required:
1. Standard Iron solution Ferric ammonium sulphate (1.726 g) dissolved in 10 ml of 0.1 N H₂SO4 and sufficient water to produce 1000 ml.
2. Sulphuric acid (0. 1 N): 10.0 ml.
3. Iron-free citric acid solution (20% w/v): 2.0 ml.
4. Thioglycolic acid: 0.1 ml.
5. Iron-free ammonia solution: 20 ml.
Chemicals Required:
1. Standard Iron solution Ferric ammonium sulphate (1.726 g) dissolved in 10 ml of 0.1 N H₂SO4 and sufficient water to produce 1000 ml.
2. Sulphuric acid (0. 1 N): 10.0 ml.
3. Iron-free citric acid solution (20% w/v): 2.0 ml.
4. Thioglycolic acid: 0.1 ml.
5. Iron-free ammonia solution: 20 ml.
Procedure for limit test for iron:
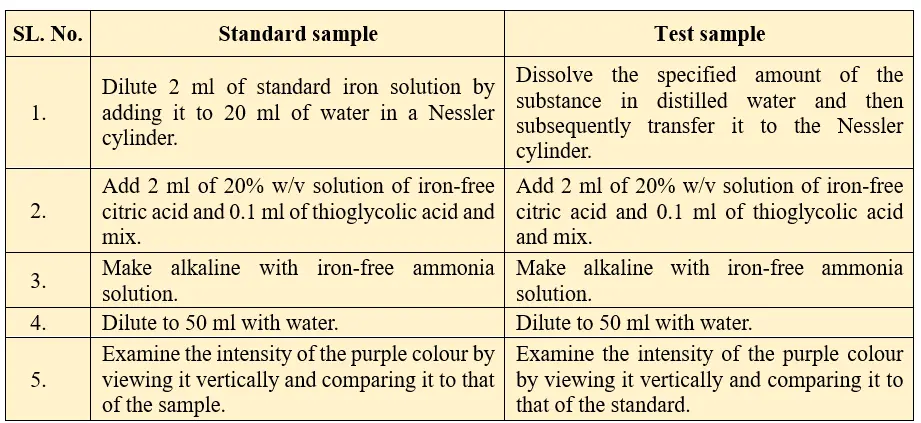
Observation:
The intensity purple color produced in the sample solution should not exceeds that of the standard solution. Intensity of the colour less than the standard solution indicates pass the limit test and intensity of the colour more than the standard solution indicates fail the limit test for iron.
Reasons:
Citric acid aids in the precipitation of iron by ammonia through the formation of a complex with it.
Thioglycolic acid facilitates the oxidation of iron (II) to iron (III).
Ammonia to make solution alkaline.
Different methods employed for limit test for Iron:
Following are the methods that can be used to perform a limit test for iron. These are-
