Definition of limit test:
Specificity of the limit test:
Any test used as a limit test, of necessity, give some form of selective reaction with the trace impurity. Many test used for the detection of inorganic impurities in official inorganic chemicals are based upon the separations involved in inorganic qualitative analysis.
Sensitivity of the test:
The degree of sensitivity needed for a limit test can greatly vary depending on the purity standard specified in the monograph. The sensitivity of most test is dependent upon a number of variable factors concentration of the solute, precipitating reagent, duration of the reaction, temperature and nature and concentration of other substances unavoidably present in solution.
Method use for limit test:
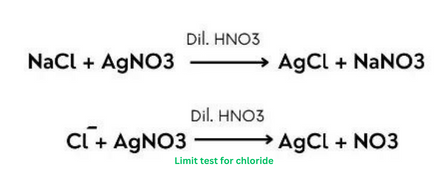
Principle of limit test for chloride:
Limit test for chloride based on the interaction between soluble chloride and silver nitrate in the presence of dilute nitric acid, resulting in the formation of solid silver chloride particles that manifest as opalescence in the solution.
Apparatus and chemicals used in limit test for chloride:
Apparatus required:
a. Nessler cylinders
b. Glass rod
c. Stand
Chemicals required:
a. Dilute Nitric acid (10%)
b. Silver nitrate (5%)
c. Sodium chloride
Nessler Cylinder:
Nessler cylinders are laboratory test tubes with a fixed volume, made of glass with optically plane bottom. The walls bear markings denoting the nominal stroke volume, typically set at 100 ml, and potentially a halfway mark, commonly indicating 50 ml.
Procedure:
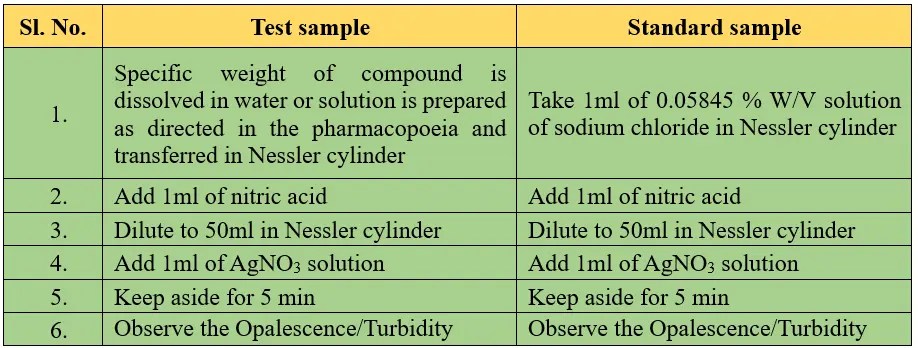
Reasons:
In the limit test for chloride, nitric acid is introduced to acidify the solution, facilitating the precipitation of silver chloride, which ultimately induces turbidity in the solution.
Results and conclusion:
If the opalescence produced in the sample solution exceeds that of the standard solution, the sample fails the chloride limit test; conversely, if the opalescence in the sample solution is less than that of the standard solution, the sample passes the test.
For comparison the turbidity for different substances with varying amount of impurity, the amount of substance to be used is varied and not the standard turbidity. Pharmacopoeias do not give a numerical value to the limits, as it is not practicable as its content will be influenced to great extent, by large quantities if other substances present.
Also read: Limit test for arsenic.
Also read: Limit test definition
