The Significance of Limit Testing:
Limit testing involves determining the maximum allowable concentration of a substance, beyond which it poses a risk to human health or the environment. For arsenic, establishing these limits is crucial due to its widespread presence and harmful effects. Chronic exposure to even low levels of arsenic can lead to various health issues, including skin lesions, cardiovascular disease, and cancer.
In the context of food and water safety, regulatory bodies worldwide have set stringent limits for arsenic levels to protect consumers. These limits are based on extensive research and risk assessments, taking into account the toxicity of arsenic compounds and their potential health effects. By conducting limit testing, authorities can ensure that products intended for human consumption meet these safety standards, thereby minimizing the risk of arsenic-related health problems.
Principle:
According to pharmacopeial guidelines, the limit test for arsenic, commonly known as the Gutzeit test, relies on the interaction between arsenic gas and hydrogen ions. This reaction occurs in the presence of reducing agents like potassium iodide, resulting in the formation of a yellow stain on mercuric chloride paper. When this gas comes into contact with mercuric chloride paper, it induces a color change, ranging from yellow to brown. The intensity and duration of this color alteration directly correlate with the concentration of arsenic present in the sample.
Reaction:
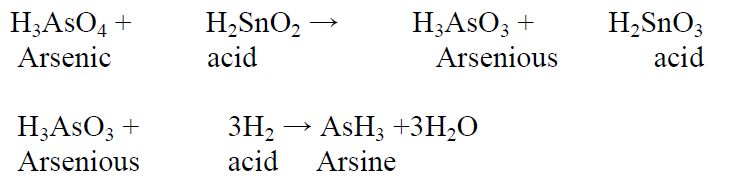
Reagents required in the limit test for arsenic:
All the specific reagents used in the limit test for arsenic are designated and identified by the letter “as T.” This designation indicates that these reagents must be free from arsenic impurities and must themselves meet the criteria outlined in the limit test for arsenic.
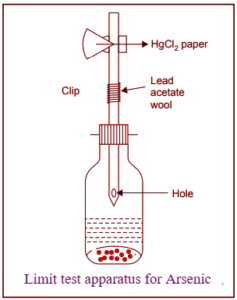
1. Stannous chloride is used to facilitate the complete release of arsine gas.
2. Zinc, potassium iodide and stannous chloride are used as a reducing agent in this process.
3. Hydrochloric acid is used to render the solution acidic.
4. Lead acetate pledgers or papers are used to trap any hydrogen sulphide that might be produced concurrently with arsine gas.
Precaution to be taken in the limit test for arsenic:
1. If the stain present in the filter paper becomes dark, the test should be repeated by using pure reagent.
2. The most suitable temperature fir carry out the test is 40 oC.
3. Care must be taken that the filter paper remains quite dry during reaction.
4. During succeeding test the tube must be washed with HCl AsT, rinsed with water and dried.
5. All the reagents used for the test should be free from arsenic and mentioned as AsT.
Procedure:
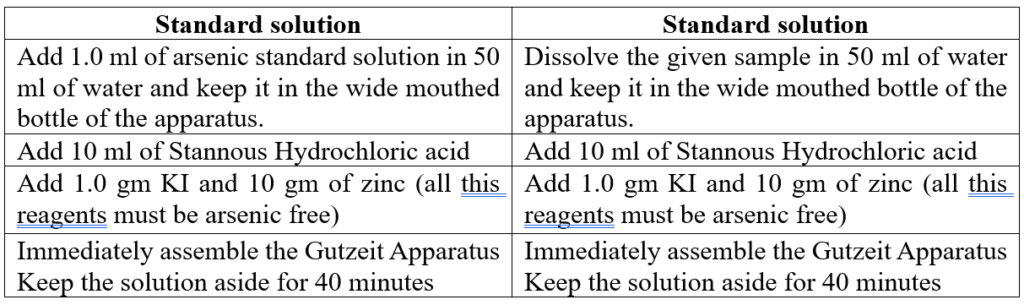
Result:
Related post: Limit test definition
Related post: Limit test chloride.
Also read: Data Integrity in Pharma:: Issue, Solutions and Challenges in Pharma Manufacturing.
