1.0 PURPOSE:
To lay down a procedure for handling of external laboratory analysis or contract laboratory tests results which are not performed in-house.
2.0 SCOPE:
3.0 DEFINITIONS:
Nil.
4.0 RESPONSIBILITY:
4.1 Quality Control Analyst is responsible for sending sample to External Testing Laboratory.
4.2 Head – Quality Control or his designee to ensure compliance.
5.0 PROCEDURE:
5.1 Procedure for sending sample to External Testing Laboratory / Contract Laboratory:
5.1.1 Identify the test(s) which is not possible to perform at In-house facility
5.1.2 Calculate the sample quantity is required to perform the particular test(s) and pack it properly.
5.1.3 Fill the analysis request form.
5.1.4 The analysis request form numbering should be ETS/XXXX/YY.
Whereas,
ETS: represents External Testing Sample
XXXX: represents the serial no starting from 0001
YY: represents the current year
5.1.5 The sample should be labeled properly.
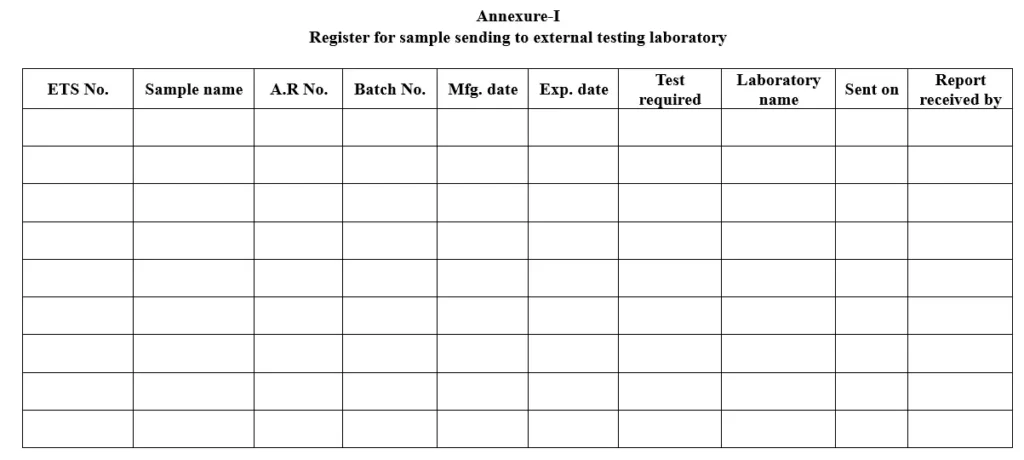
5.1.6 Enter the details of the sample testing for external laboratory in the register as per annexure QCD/GEN/025/A02.
5.1.7 Review the report received from the external laboratory with respect to the specification and put the reviewed by stamp with signature on the same report.
5.1.8 Update the same in the external testing laboratory register.
6.0 ABBREVIATIONS:
7.0 REFERENCES:
8.0 LIST OF ANNEXURES: