Definition of Good laboratory practices (GLP):
Good Laboratory Practices (GLP): Good Laboratory Practices is a quality system comprises with the organizational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, reported and Archived.
GLP, or Good Laboratory Practices, is a regulatory framework established by the USFDA. These regulations were first proposed on November 19, 1976, and officially designated as a new part of Chapter 21 of the Code of Federal Regulations (CFR) in 1979, specifically as 21 CFR Part 58.

The purpose of Good laboratory practices (GLP):
The fundamental aim of Good Laboratory Practices (GLP) is to enhance the quality and reliability of test data used in determining the safety of various substances such as chemicals, pharmaceuticals, food, cosmetics, and more.
Importance of Good laboratory practices (GLP):
Advantages and Disadvantage of GLP:
1. Assures that the data are a true reflection of results obtained from studies.
2. Preclinical safety and residue safety.
3. Generation of high quality and reliable test data.
4. Mutual acceptance of data
5. Increases public confidence.
6. Shortens the time-to-market for new products.
Disadvantages of Good laboratory practices (GLP):
1. More manpower is required.
2. Expensive process.
3. Time consuming process.
Objectives of Good laboratory practices (GLP):
1. Good laboratory practices (GLP) ensures that the data submitted are true reflection of the results obtained from the studies.
2. Good laboratory practices (GLP) ensures sure that the data is traceable.
3. Promotes international acceptance of tests.
The elements of Good Laboratory Practices:
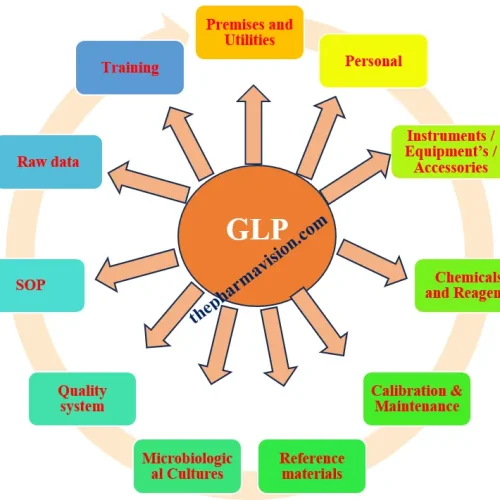
1.0 General Requirement:
1.1 Good laboratory practices concern all people who work in areas where it applies, whether they are managers, group leaders or other personal. Although persons working in GLP environment is carrying out different tasks using different skills, there are some basic rules which can be generally applied are follows:
1.2 Make sure to have the correct written instructions before starting a task.
1.3 Do not carry out a task for which user have not been trained or in which user do not feel competent.
1.4 Always follow instructions precisely.
1.5 Define in advance what we are going to do.
1.6 Check that what have done and what shall have to be done.
1.7 Keep records of information, results and actions taken. Maintain clear accurate records of the activity what was done and the checks carried out.
1.8 Check that the instruments / equipment’s used are clean, calibrated and correct ones as per procedure before use.
1.9 Always be on guard for labeling errors.
1.10 Labels shall always be held securely and not left lying around.
1.11 Always notify if labels are seen either detached or appear to be incorrect or are in wrong place.
1.12 Report any labels that are damaged or dirty.
1.13 Never remove a label which has been incorrectly applied and never stick a new label over an old one of the same type. Incorrectly affixed should be strike it off with sign and date and paste new correct label adjacent to it.
1.14 Ensure that printed labels which are used are legible; if not then replace the same. The labels with poor legibility shall be “X” marked with marker pen and discarded by tearing wherever possible.
1.15 Keep everything clean and tidy.
1.16 Investigate problems. Always be on the lookout for mistakes, defects and unusual events. Report them immediately.
1.17 Quality Control Laboratory shall be clean and the workbenches kept tidy in accordance with written cleaning schedules and whenever it is required.
1.18 The cleaning and disinfection of microbiological testing areas shall be regular and according to written schedules and procedures.
1.19 The cleaning of Raw Material, Packaging Material, Finished Product and Stability samples storage compartment is to be done by using vacuum cleaner (if required) followed by cleaning with soft lint free duster. During cleaning avoid any possible cross contamination of the samples.
1.20 Clean the instruments / equipment’s like Glassware drying oven, Refrigerator, Deep freezer, Water bath, Sonicators, etc., used in the Quality Control Laboratory fortnightly or earlier as and when required.
1.21 Clean the workbenches after completion of work or at the end of the day whichever is earlier and keep the respective specification, GTP’s, SOP’s etc. used, back at the designated place.
1.22 General and specific written down instructions for safety shall be circulated to each staff member and the instructions shall be revised periodically as appropriate (e.g. audio-visual material, poster displays and by seminars/conferences).
1.23 While closing the Quality Control laboratory, ensure that all water taps, instruments (which are not running), equipments, computers are switched ‘OFF’. Put off the lights, AC’s and inform the Engineering department to put off the AHU’s (wherever required) and close the department.
1.24 The laboratory main door keys shall be submitted to Security department.
2.0 Premises and Utilities:
2.1 Premises must be built and maintained to suit the operations being carried out. Separate areas shall be provided wherever required e.g. chemical, microbiological, instrumental analysis etc.
2.2 Interior surface (walls, floor, and ceilings) shall be smooth and free from cracks, and permit easy cleaning and disinfection.
2.3 Adequate provision shall be made not only for space and equipment for carrying out necessary test but also for utilities like water, electrical power and gas.
2.4 Air ventilation system shall ensure dust free environment.
2.5 The laboratory shall be provided with adequate light and ventilation and if required air-conditioning to maintain satisfactory temperature and relative humidity.
2.6 Tabletops shall be constructed with acid, alkali, and solvent resistant material and shall be smooth.
2.7 There shall be adequate and suitable storage space for samples, standards, laboratory reagents, apparatus, accessories and records.
2.8 Appropriate measures shall be established and implemented to prevent cross-contamination from personnel, materials etc.
2.9 The utilities such as electricity, compressed air, gas required for the functioning of laboratory shall be adequate.
2.10 The cleaning and sanitation of washing area and drainage points shall be done as per procedure.
2.11 Monitor the room condition with automated electronic device at periodic interval as specified.
2.12 Adequate storage area / facility with lock and key shall be provided wherever protection is necessary to avoid misuse or hazard like poisonous material, etc.
2.13 The eye washer, safety shower should be in working condition and Laboratory shall be provided with first aid kit for any emergency which may arise during operation. Similarly the required fire extinguisher shall be available in the laboratory to extinguish any fire in case of emergency
2.14 An emergency exit shall be available (shall be free from obstacles) at appropriate place for exit during emergency.
3.0 Personal:
3.1 There shall be adequate number of personnel qualified with appropriate education, training and / or experience to perform and supervise the tasks assigned. A laboratory shall have a Head (Quality Manager or Technical Manager) for carrying out all technical activities and for implementation of documented quality system.
3.2 Head of the laboratory must be of high professional standing with experience in drug analysis and laboratory management who is responsible for:
3.2.1 Ensuring the control and maintenance of documents including the quality system as per the requirements of regulatory authorities which involve all raw data, SOP’s, documentation exhibits, protocols, training charts, etc.
3.2.2 Planning and organizing the audit of the quality system and initiation as well as follow up action of the corrective actions, if any.
3.2.3 Investigation of technical complaints.
3.2.4 Taking final responsibilities for recommending any regulatory action in the event of noncompliance of tested samples.
3.3 All personnel prior to employment shall be medically examined and then periodically re-examined for medical fitness. The Head Quality Control shall ensure that the personnel are medically fit to carry out the job.
3.4 Personnel who are suffering from an infectious disease or having open lesions on the exposed surface of the body are prohibited from participating in any activities that could result in compromising the quality of analysis.
3.5 All employees shall be instructed to report about their illness or abnormal health condition to their immediate supervisor so that appropriate action can be taken.
3.6 Pyramid according to the job responsibilities and functions shall be clearly defined.
3.7 The job responsibility shall be assigned according to competency of the person and it shall be timely revised for addition or deletion of responsibilities assigned previously.
3.8 The job responsibilities of the personnel engaged shall be specified in writing and followed strictly.
3.9 Personnel shall practice good sanitation and health habits.
3.10 In laboratory areas, smoking, eating, drinking, chewing, keeping plants, food, drinks, and personal medicines, as well as installing or removing contact lenses, are strictly prohibited. Do not drink anything available in laboratory.
3.11 Personnel shall wear clean clothing (company uniform) suitable for activity with which they are involved and this clothing shall be changed on daily basis or when appropriate. Personnel shall strictly follow entry / exit and gowning procedures.
3.12 While sampling and handling cytotoxic materials, poisonous, carcinogenic materials etc., while handling hazardous chemicals and while performing microbiological analysis etc., procedure of change of clothing and use of personal protective equipments and safety appliances shall be strictly followed. Personnel shall avoid direct contact with material / product.
3.13 Never handle any chemicals, raw materials, intermediate or finished, unpacked products with bare hands. Always use appropriate hand gloves while handling the same.
3.14 Maintain discipline inside the laboratory premises.
4.0 Instruments / Equipment’s / Accessories:
4.1 The laboratory should be furnished with all types of instruments / equipment’s as may be necessary for carrying out the different activities within the laboratory.
4.2 The analytical instrument should be housed in dust-free environment whenever required. Conditions of temperature and humidity should be maintained and periodic checks on temperature and humidity should be made and recorded.
4.3 The instruments / equipment’s, instrument / equipment bench and surrounding areas should be kept clean, tidy and free from vibration at all times.
4.4 Instruments / equipment’s requiring calibration should be calibrated at regular intervals and records of such calibration or maintenance should be maintained. There should be written instructions in the form of Standard Operating Procedures for the operation and calibration wherever applicable. A log Book (day to day entry including status of the equipment) shall be maintained for all major instrument or wherever applicable.
4.5 Other equipment’s / accessories such as burettes, pipettes, volumetric flasks, weight boxes, thermometers etc. should be thoroughly checked for accuracy of calibration before acceptance for use.
4.6 During weighing excess working standard should not be placed back into the original container and should be discarded except Reference or costly material.
4.7 Standard Operating procedure should be displayed or kept near instruments/ equipment’s and should be followed whenever used.
4.8 While performing analysis on instruments / equipment’s display the analysis status indicating details of ongoing analysis.
4.9 Fume Cupboards/Fume Hood: Work involving the evolution of harmful and obnoxious vapors shall be carried out in a fume cupboard.
4.10 Each balance weight print should be clearly identified and wherever possible/applicable the balance weight prints should be stamped with Rubber stamp with minimum but not limited to the details like weight, Test, AR No./B. No. and Analyzed by.
5.0 Chemicals and Reagents:
5.1 The storage and handling of chemicals and reagents shall be done in a manner considering the physicochemical properties of these substances and the hazards involved in their use, i.e. refer chemical/reagent MSDS before handle the chemical.
5.2 All reagents and solutions in the laboratory shall be properly identified with a label.
5.3 Containers of stock solutions and of standard solutions shall bear the following details:
5.3.1 Name of analytical chemist who prepared the solution.
5.3.2 Date of preparation.
5.3.3 Each volumetric solution shall have ―use before date/valid up-to date depending upon the stability of the solution.
5.3.4 The transfer of hazardous chemicals and regents from one container to another container shall be carried out with suitable equipment by taking the care of safety.
6.0 Good housekeeping and safety:
Standard Operating Procedure for safety, house-keeping and loss prevention shall be prepared, include the following requirements:
6.1 Safety data sheets must be made available to staff before testing is carried out.
6.2 Drinking, eating and smoking shall not be permitted in the laboratories; food for human consumption shall not be kept in working or storage areas.
6.3 All personal must wear laboratory coats or other protective clothing including gloves and face masks and eye protection wherever required.
6.4 Rubber suction bulbs must be used on manual pipettes and siphons.
6.5 Warnings, precautions, and written instructions must be given for work with violent, uncontrollable or dangerous reactions (e.g. mixing acids and water, biological such as infectious agents, etc.)
6.6 Appropriate facilities for the collection, storage, and disposal of wastes shall be made available.
6.7 All personal must be aware of methods for safe disposal of corrosive or dangerous products by neutralization or deactivation and of the need for complete disposal of mercury and its salts.
6.8 All personal must also be aware about the safety precautions to be adopted while handling poisonous material such as potassium cyanide and cyanogen bromide.
7.0 Calibration and Maintenance:
7.1 Each instrument / equipment should have calibration status tag; indicating date of calibration. due date for next calibration with sign and date .Calibration status should be ensured for validity prior to use.
7.2 All instruments / equipment’s should be calibrated as per defined schedule and as per procedure. The records of calibration should be maintained. The standards used for calibration should have traceability with national or international standards (as applicable).
7.3 The maintenance of equipment’s for services like electricity, gas, water, steam and compressed gas should be handled by competent person.
7.4 If any Instrument / Equipment shows malfunctioning, impact assessment on previous analysis should be done.
8.0 Reference materials:
8.1 The laboratory shall prepare working standard by comparing with the reference standards.
8.2 A register pertaining to reference and working materials must be maintained by the laboratory. The register should contains the following details:
8.2.1 Source of supply.
8.2.2 Date of receipt.
8.2.3 Batch number or identification number of the supplying agency.
8.2.4 Details like assay value, water content or any other information provided.
8.2.5 Storage condition of the material.
8.2.6 Date of expiry, if any and date of manufacturing if possible.
9.0 Microbiological Cultures:
9.1 Standard Operating Procedure for maintenance of microbial culture and sub-culture must be prepared by the laboratories.
9.2 If the cultures have become non-viable or mutant, proper procedure shall be followed to destroy these cultures by autoclaving under an authorized personnel for biological testing.
9.3 All activities be carried out in a aseptic area by authorized person.
9.4 The laboratories shall perform standard biochemical tests on the sub-culture as given in literature to ensure their viability.
10.0 Quality system:
10.1 The measurements and calibrations shall fully conform to the compendial requirements and the methods demonstrably based on validation protocols are followed.
10.2 Remedial action on the observations by internal and external audits are taken appropriately.
10.3 There must be comprehensive and effectively implemented Quality Control systems which shall be fully documented and their effectiveness shall be monitored.
10.4 Quality Control Personnel shall have access to Production and Warehouse areas for sampling and investigation as appropriate.
10.5 Quality Control shall ensure that every product it manufactures and distributes, consistently meets with standards of Quality, Purity, Efficacy and Safety.
10.6 Quality Control is responsible for fulfilling the regulatory requirements for product registration.
10.7 Organogram is to be prepared for the Quality Control department.
11.0 Standard Operating Procedures:
11.1 Standard Operating Procedures are written procedures for different activities and are integral part of Good laboratory Practices.
11.2 Testing laboratories shall have Standard Operating Procedure manuals and have its periodic review.
11.3 It shall be user friendly documents and shall include designation of the person responsible for intended activity.
12.0 Raw data:
12.1 Raw data refers to the laboratory work sheet, analysis sheet, records, and other activities and such raw data shall include hand written notes, photographs, software, drawings, computer printouts, spectral charts etc.
12.2 A single line shall strike through the data being changed; the correct information shall be recorded along with the old data and the reason of change. The analyst making the change shall be identified by his signature with date. In case of automated data collection system, the person responsible shall be identified at the time of data output. The original entry must be saved and the system have audit trial for all the data.
12.3 Raw data refers to the laboratory work sheet, analysis sheet, records, and other activities
12.4 Data integrity and security shall be maintained and the data shall not be accessible to any unauthorized person.
13.0 Training:
13.1 Training shall be regularly conducted by qualified individuals and shall cover, at a minimum, the particular operations that the employee performs and GMP as it relates to the employee’s functions. GLP / GMP ultimately depend on people. Training shall be given on both the theory and practice of the work being undertaken in a particular area, as well as relevant ‘on-job’ (i.e. task-based) training.
13.2 Records of training must be maintained. Training shall be periodically accessed. All staff, including new and existing shall be given basic training on ‘Good Laboratory Practices’ during induction and at regular intervals subsequently. These training programs shall be periodically updated. In addition during training, emphasis shall be laid on regulatory requirements, health, safety and hygiene.
14.0 Documentation:
14.1 Clearly written documentation prevents errors may occur in verbal communication.
14.2 Specifications, instructions, procedures and records must be free from errors and available in writing. Standard operating procedures should be available for sampling, inspection and testing of raw materials, API’s, intermediates, bulk finished products, finished products, stability samples, packaging materials and whenever necessary.
14.3 Documents should be approved, signed and dated by appropriate and authorized persons.
14.4 On receipt of certificates from outside location / organization (such as. Working standard certificate, approval External party calibration certificate, certificate of analysis of raw material received from supplier etc.) the same should be reviewed and verified by the quality control personal with sign.
15.0 Desiccant Use and Replacement:
15.1 Sufficient quantity of self-indicative silica gel or molecular sieve shall be used, Wherever the control on absorption of moisture is required during operation or measurement, e.g. analytical balances where exposure of hygroscopic material during weighing will result absorption of moisture and in-appropriate weighing amount.
15.2 In Quality Control department the instruments / areas like Analytical Balance, Autotitrator, Karl Fischer Titrator, FTIR, UV Spectrophotometer, Desiccators, Karl Fischer Coulometer, Gas manifold needs use of desiccant and routine monitoring of replacement of the same.
15.3 Before using any instrument / equipment, check the color of silica gel / rubin gel used as desiccant for the instrument and equipment.
15.4 Also the desiccant used in the different desiccators is to be replaced if desiccant gets deactivated (Blue color faded to pink / colorless). Maintain the record of the desiccant replacement.
15.5 Replace the used desiccant with the new or regenerated desiccant.
16.0 Handling of instruments:
16.1 Ensure that the instruments are qualified before use.
16.2 Ensure that instruments are calibrated and maintained as per the respective calibration procedure and schedule. Calibration shall be performed as per the schedule.
16.3 The calibration status of the instrument shall be displayed on the instrument.
17.0 Handling of Spillage:
17.1 Sample, solid chemical and broken glassware Spillages:
17.1.1 Clean the Spillages of analytical samples (such as Tablets, Capsules, Raw material) solid chemicals and broken glassware by using suitable broom or wet mop cloth.
(Broken glassware and their particles shall be collected carefully, do not collect the particles directly with bare hands).
17.1.2 Collect the particles/samples in specified container.
17.1.3 Clean the place by wet moping followed by dry moping.
17.2 Solvent and hazardous chemicals Spillages:
In case of any spillages of solvent or hazardous chemicals below method shall be used:
17.2.1 For Hazardous chemical first neutralized it by using 0.1 N sodium hydroxide/ diluted hydrochloric acid.
17.2.2 If the chemical having strong odour such as ammonia or acetic acid then used flavored solved such as detol or sevlon to mask the odour.
17.2.3 Clean the spillages of solvent by dry moping.
17.2.4 In case of solvent spillages is near about hot zone area, switch off the instrument nearby to avoid any accident.
17.2.5 While handling these spillages always use personal PPE (Gloves, Mask, apron and safety shoes).
17.2.6 Wash the area with plenty of water.
17.2.7 Clean the water with dry moping.
17.3 Water Spillages:
Always use dry mop to clean water spillages.
18.0 ‘Do’s inside the QC laboratory:
18.1 The use of correct and current specifications and STP.
18.2 Timely recording of raw data in the issued work sheets as well in log books.
18.3 Checking of the instrument calibration validity before used.
18.4 Data is written with proper cross reference, wherever required.
18.5 Immediate reporting of the Out of specification results, out of trend results, or any abnormalities observed to the superior.
18.6 Use of clean and dry glassware for analysis.
18.7 Label the glassware with minimum details like Product name, batch number, test name, dilutions (if any) and analyst sign and date for identification and tractability. For small glassware labeled as much as possible details.
18.8 Use of fume hood for odour/fume releasing materials.
18.9 Handling of hazardous materials as per Standard operating procedure (SOP).
18.10 Rectification of writing errors – cross out with signature and date.
18.11 Use personal protective equipment wherever possible.
18.12 Adjust the level up to the mark means “Make up the level with liquid up to lower meniscus for colorless solutions and upper meniscus for colored solution.
18.13 Fill the HPLC vial up-to 80 % of its capacity.
18.14 Use amber color glassware including HPLC vials for analysis as per requirement.
18.15 Keep vials into respective system as soon as possible after filling vials with solutions.
18.16 Precaution to be taken during related substance/degradation product analysis: Use designated or separate place (as possible) to avoid contamination. Also ensure the cleanness of glassware, analyst hand/gloves etc. before analysis. Rinse the vials with sample solution to be filled for 5 times before vial filling and glassware with diluent before analysis.
18.17 Store all the remaining preparations as recommended in standard test procedure till completion of analysis.
19.0 ‘Dont’s inside the QC laboratory:
19.1 Use of loose paper sheets for writing the observations.
19.2 Blowing of pipettes by mouth.
19.3 Use of broken glassware for analysis.
19.4 Use of eraser or white ink.
