1.0 PURPOSE:
To lay down a procedure for management of water quality.
2.0 SCOPE:
This procedure for management of water quality is applicable for sampling, testing, approval and rejection of different types of water used at manufacturing premises of (Company name).
3.0 DEFINITIONS:
Nil.
4.0 RESPONSIBILITY:
4.1 Analyst-for sampling and testing of water samples.
4.2 Sectional head/Group Leader—for implementation and technical compliance of this SOP.
4.3 Head-production/Head-engineering or their designee shall participate in investigation if there is out of specification result & if the result is beyond alert and action limit.
4.4 Department Head/Head quality Assurance or their designee to ensure compliance of the SOP.
5.0 PROCEDURE:
5.1 Sample Containers Preparation:
5.1.1 For Chemical analysis:
5.1.1.1 Wash a glass bottle of desired volume, rinse with potable water and finally rinse with purified water. Dry the bottle in oven at 60°C.
5.1.2 For Microbiological Analysis:
5.1.2.1 Use autoclavable, screw capped glass bottle of desired volume, rinse the bottle with purified water. Dry the bottle in oven at 60°C.
5.1.2.2 Cap the bottle securely and autoclave as per validated sterilization cycle.
5.1.3 For Bacterial Endotoxin Test:
5.1.3.1 Wash the test tube/bottle with purified water. Wrap the test tube/bottle with double layer of aluminium foil and depyrogenate in hot air oven as per validated depyrogenation cycle or use ready to use depyrogenated glass tube/bottle. Use this tube/bottles to collect Water For Injection and pure steam condense for bacterial endotoxin testing.
5.2 Sampling of water:
5.2.1 Label all the water sample containers properly before sampling.
5.2.2 Take sample containers to respective location in a sampling kit/sampling tray.
5.2.3 Collect water samples from various sampling points in separate containers for chemical analysis, microbiological analysis, TOC analysis and endotoxin analysis, as applicable.
5.2.4 Collect sample for Microbiological Analysis first, followed by Bacterial Endotoxin Test, TOC Analysis and Chemical Analysis, as applicable.
5.2.5 If any extension / hosepipe is attached to sampling point and is used during routine water usage, collect water sample at the end of extension / hosepipe.
5.2.6 Hold sterile bottle near the valve, unwrap the aluminium foil / sterilization paper and open the bottle taking care not to touch the cap or neck of the bottle.
5.2.7 For microbiology analysis; Collect specified volume of water sample in the bottle without rinsing and immediately close it with the cap. Do not fill the bottle up to the neck.
5.2.8 Immediately after this, collect water sample for bacterial endotoxin test, if applicable, in de-pyrogenated test tube/bottle and close with aluminium foil.
5.2.9 Now, collect water for TOC analysis, if applicable.
5.2.10 For TOC analysis sample, rinse the container 2 to 3 times with water to be collected from the sampling point and collect desired volume of sample in bottle/auto sampler vial and tightly close the container allowing no/minimal headspace.
5.2.11 Meanwhile continue the water to run through the valve.
5.2.12 For chemical analysis sample, rinse the container 2 to 3 times with water to be collected from the sampling point and collect specified volume of sample in the bottle and close the valve after collecting sample.
5.2.13 Bring all the samples safely in SS sampling kit to laboratory to avoid any contamination during transportation.
5.2.14 Analyze the samples for microbial tests within 2 hours after sampling.
5.2.15 In case if the samples are not taken for analysis, then store samples in refrigerator at 2°to 8°C immediately after sampling until analysis.
5.3 Analysis of water:
5.3.1 Keep the sample at room temperature before starting analysis to attain room temperature.
5.3.2 Perform the analysis of all types of water as per current version of specifications and Standard Test Procedure (STP).
5.3.3 Analyze pure steam generator outlet as per current version of Water for Injection specification and STP except microbiology testing. (i.e., Total microbial count and test for specified microorganisms).
5.3.4 Analyze other user points of pure steam (condensate) for description, pH, conductivity, TOC and bacterial endotoxins as per water for injection specification.
5.3.5 Analyze return and outlet of tank of purified water, water for injection for the entire test mentioned in the STP.
5.3.6 Analyze all other user points of purified water and water for injection, for test of Description, pH, TOC, conductivity and microbiological tests.
5.3.7 Perform the potable water analysis as per the schedule.
5.3.8 If there is a holiday in any of the day as per sampling schedule, the samples of those points shall be collected on the previous working day itself. If the holiday is unscheduled than the samples to be collected on the next working day.
5.3.9 Prepare trend charts of analytical results periodically.
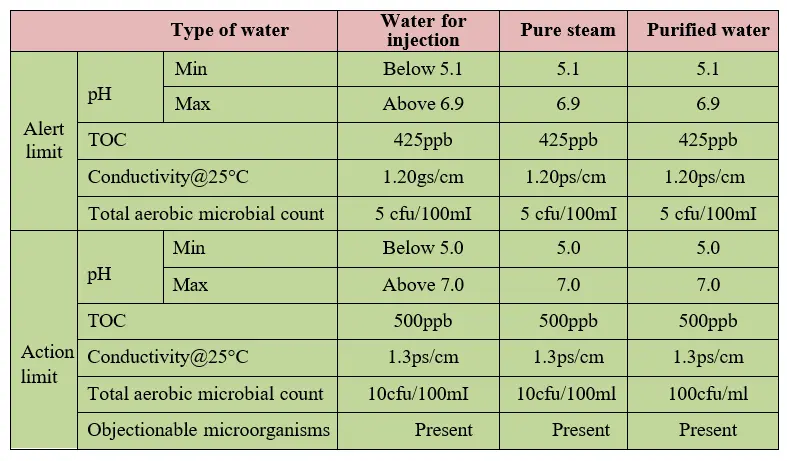
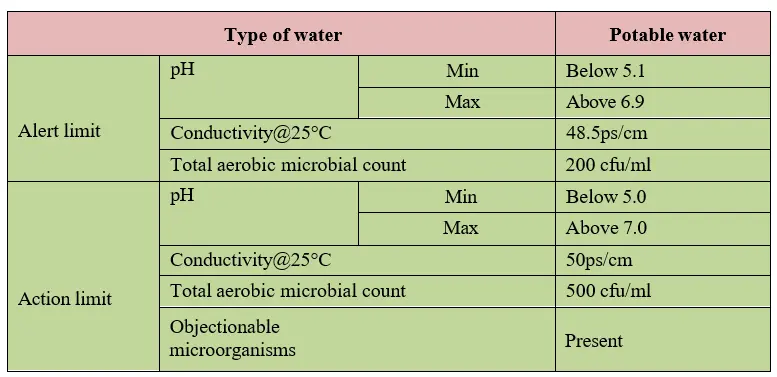
5.4 Alert and action limit:
5.4.1 An alert and action limit for WFI, pure steam and purified water is mentioned as below.
6.0 ABBREVIATIONS:
SOP : Standard Operating Procedure
TOC : Total Organic Carbon.
CFU : Colony Forming Unit
SS : Stainless steel.
7.0 REFERENCES:
Nil.
