Principle:
The limit test for Lead (Pb) relies on a reaction between lead impurities and Diphenylthiocarbazone (Dithizone) in a chloroform solution under alkaline conditions. This reaction forms a Lead-dithizone complex, which exhibits a red coloration.
In its natural state, Dithizone appears green in chloroform. However, when it forms a complex with lead, the resulting compound exhibits a violet color. Consequently, the color observed at the end of the process is red.
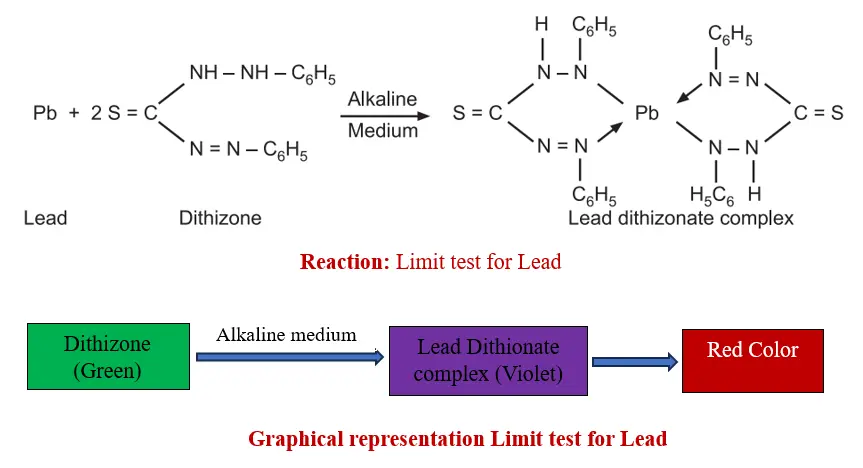
Dithizone in chloroform, extract lead from alkaline aqueous solution as lead dithizone complex (violet in color).
In this experiment, ammonium citrate, potassium cyanide, hydroxylamine hydrochloride are used to extract and discard and interfering metal ions (other than lead) at optimum pH in the form of complex.
Procedure for limit test for Lead:
Dithizone in chloroform, extract lead from alkaline aqueous solution as lead dithizone complex (violet in color).
In this experiment, ammonium citrate, potassium cyanide, hydroxylamine hydrochloride are used to extract and discard and interfering metal ions (other than lead) at optimum pH in the form of complex.
Sr. No. | Test sample | Standard sample |
1. | A specified quantity of sample solution is transferred into a separating funnel | A standard lead solution is prepared equivalent to the permissible amount of lead in the sample under examination. |
2. | Add 6 ml of ammonium citrate | Add 6 ml of ammonium citrate |
3. | Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride | Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride |
4. | Add 2 drops of phenol red | Add 2 drops of phenol red |
5. | Make solution alkaline by adding ammonia solution | Make solution alkaline by adding ammonia solution |
6. | Extract with 5 ml of dithizone until it becomes green | Extract with 5 ml of dithizone until it becomes green |
7. | Combined dithizone extract are shaken for 30 mins with 30 ml nitric acid and chloroform layer is discarded | Combined dithizone extract are shaken for 30 mins with 30 ml nitric acid and chloroform layer is discarded |
8. | To the acid solution add 5 ml of standard dithizone solution | To the acid solution add 5 ml of standard dithizone solution |
9. | Add 4 ml of ammonium cyanide | Add 4 ml of ammonium cyanide |
10. | Shake for 30 mins and observe the colour | Shake for 30 mins and observe the colour |
Notes:
Ammonium citrate, potassium cyanide, and hydroxylamine hydrochloride are employed to establish the optimal pH, thereby eliminating interference and the influence of other impurities.
Phenol red serves as an indicator to facilitate the development of color at the end of the process.
Observation:
Related post: Limit test for sulphate.
Related post: Limit test for Iron.
Related post: Limit test for chloride.
Related post: Limit test for arsenic.
